Browser
Protein data
function: electron transporterexperiment: ELECTRON MICROSCOPY
resolution: 2.1 Å
origomeric count: 22
axial ligand #1: HIS
chainID: 3P,resSeq: 97,
Coordination distance[Å]: 2.066
molecule: Cytochrome b
Organism: Sus scrofa
axial ligand #2: HIS
chainID: 3P,resSeq: 196,
Coordination distance[Å]: 2.535
molecule: same as ligand #1
List of other hemes in pdb:8ugk
| ID of heme | Distortion | Axial ligands on heme | Function & structure | |
|---|---|---|---|---|
8ugk-3C-501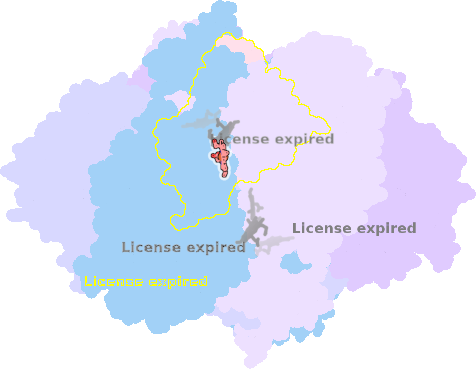 |
sad. +0.08 ruf. -0.19 dom. +0.06 bre. +0.02 |
HIS | chainID: 3C, resSeq: 83, molecule: Cytochrome b |
electron transporter oligomeric count: 22 pocket vol.: 404.0 Å3 d(Fe-oop): 0.047 Å |
| HIS | chainID: 3C, resSeq: 182, molecule: Cytochrome b |
|||
8ugk-3C-502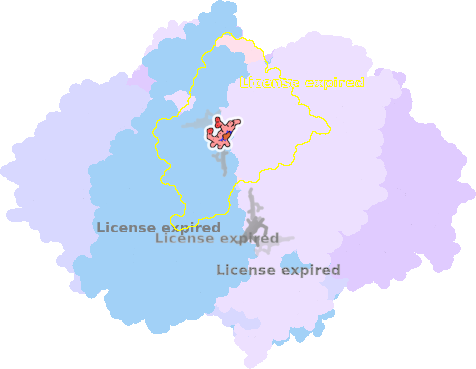 |
sad. -0.62 ruf. -0.56 dom. +0.33 bre. +0.07 |
HIS | chainID: 3C, resSeq: 97, molecule: Cytochrome b |
electron transporter oligomeric count: 22 pocket vol.: 401.0 Å3 d(Fe-oop): 0.077 Å |
| HIS | chainID: 3C, resSeq: 196, molecule: Cytochrome b |
|||
8ugk-3D-501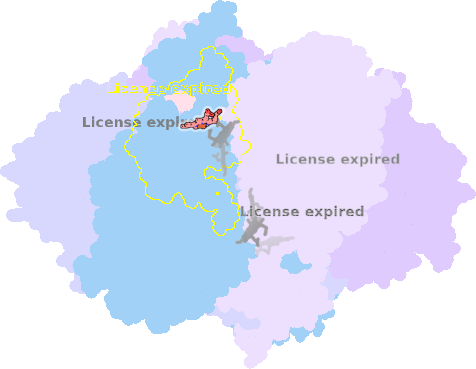 |
sad. +0.01 ruf. -0.32 dom. +0.36 bre. -0.61 |
HIS | chainID: 3D, resSeq: 129, molecule: Cytochrome c1, heme protein, mitochondrial |
electron transporter oligomeric count: 22 pocket vol.: 443.0 Å3 d(Fe-oop): 0.129 Å |
| MET | chainID: 3D, resSeq: 249, molecule: Cytochrome c1, heme protein, mitochondrial |
|||
8ugk-3P-501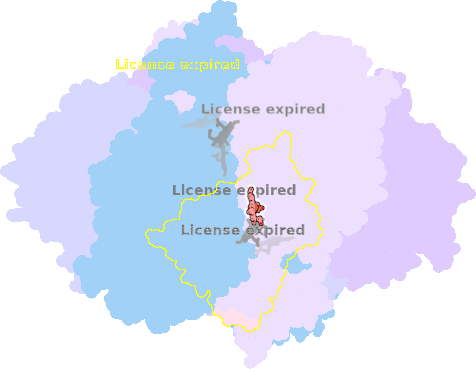 |
sad. +0.09 ruf. -0.22 dom. -0.23 bre. +0.00 |
HIS | chainID: 3P, resSeq: 83, molecule: Cytochrome b |
electron transporter oligomeric count: 22 pocket vol.: 438.0 Å3 d(Fe-oop): 0.066 Å |
| HIS | chainID: 3P, resSeq: 182, molecule: Cytochrome b |
|||
8ugk-3Q-501 |
sad. +0.00 ruf. -0.46 dom. +0.41 bre. -0.60 |
HIS | chainID: 3Q, resSeq: 41, molecule: Cytochrome c1, heme protein, mitochondrial |
electron transporter oligomeric count: 22 pocket vol.: 443.0 Å3 d(Fe-oop): 0.150 Å |
| MET | chainID: 3Q, resSeq: 160, molecule: Cytochrome c1, heme protein, mitochondrial |
|||
