Browser
Protein data
function: electron transporterexperiment: ELECTRON MICROSCOPY
resolution: 2.13 Å
origomeric count: 18
axial ligand #1: HIS
chainID: A,resSeq: 86,
Coordination distance[Å]: 2.599
molecule: Cytochrome b6
Organism: Spinacia oleracea
axial ligand #2: HIS
chainID: A,resSeq: 187,
Coordination distance[Å]: 2.565
molecule: same as ligand #1
List of other hemes in pdb:7zyv
| ID of heme | Distortion | Axial ligands on heme | Function & structure | |
|---|---|---|---|---|
7zyv-A-302 |
sad. -0.80 ruf. -0.44 dom. +0.10 bre. -0.13 |
HIS | chainID: A, resSeq: 100, molecule: Cytochrome b6 |
electron transporter oligomeric count: 18 pocket vol.: 521.0 Å3 d(Fe-oop): 0.066 Å |
| HIS | chainID: A, resSeq: 202, molecule: Cytochrome b6 |
|||
7zyv-A-303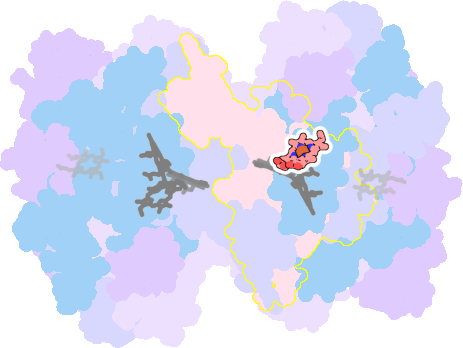 |
sad. +0.64 ruf. +0.02 dom. -0.13 bre. -0.22 |
Not assigned. | unclassified oligomeric count: 18 pocket vol.: 670.0 Å3 d(Fe-oop): 0.120 Å |
|
| Not assigned. | ||||
7zyv-C-301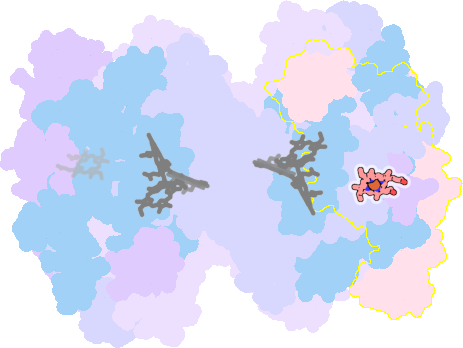 |
sad. -0.42 ruf. -0.59 dom. +0.12 bre. -0.19 |
TYR | chainID: C, resSeq: 1, molecule: Cytochrome f |
electron transporter oligomeric count: 18 pocket vol.: 461.0 Å3 d(Fe-oop): 0.078 Å |
| HIS | chainID: C, resSeq: 25, molecule: Cytochrome f |
|||
7zyv-I-301 |
sad. -0.01 ruf. +0.04 dom. +0.02 bre. -0.19 |
HIS | chainID: I, resSeq: 86, molecule: Cytochrome b6 |
electron transporter oligomeric count: 18 pocket vol.: 532.0 Å3 d(Fe-oop): 0.041 Å |
| HIS | chainID: I, resSeq: 187, molecule: Cytochrome b6 |
|||
7zyv-I-302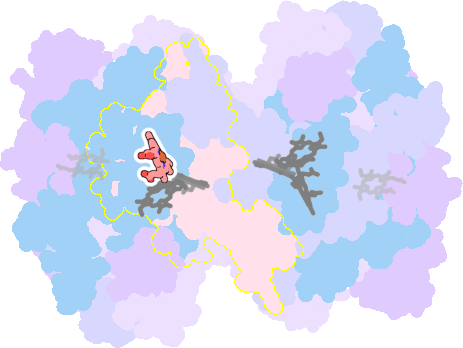 |
sad. -0.80 ruf. -0.44 dom. +0.10 bre. -0.13 |
HIS | chainID: I, resSeq: 100, molecule: Cytochrome b6 |
electron transporter oligomeric count: 18 pocket vol.: 513.0 Å3 d(Fe-oop): 0.065 Å |
| HIS | chainID: I, resSeq: 202, molecule: Cytochrome b6 |
|||
7zyv-I-303 |
sad. +0.64 ruf. +0.02 dom. -0.13 bre. -0.22 |
Not assigned. | unclassified oligomeric count: 18 pocket vol.: 660.0 Å3 d(Fe-oop): 0.120 Å |
|
| Not assigned. | ||||
7zyv-K-301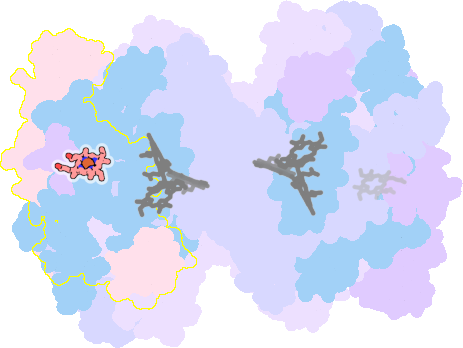 |
sad. -0.42 ruf. -0.59 dom. +0.12 bre. -0.19 |
TYR | chainID: K, resSeq: 1, molecule: Cytochrome f |
electron transporter oligomeric count: 18 pocket vol.: 469.0 Å3 d(Fe-oop): 0.078 Å |
| HIS | chainID: K, resSeq: 25, molecule: Cytochrome f |
|||
