Browser
Protein data
function: oxidoreductaseexperiment: X-RAY DIFFRACTION
resolution: 2.6 Å
origomeric count: 24
axial ligand #1: MET
chainID: G,resSeq: 52,
Coordination distance[Å]: 2.33
molecule: BACTERIOFERRITIN
Organism: ESCHERICHIA COLI
axial ligand #2: MET
chainID: H,resSeq: 52,
Coordination distance[Å]: 2.368
molecule: BACTERIOFERRITIN
Organism: ESCHERICHIA COLI
List of other hemes in pdb:3e1q
| ID of heme | Distortion | Axial ligands on heme | Function & structure | |
|---|---|---|---|---|
3e1q-A-200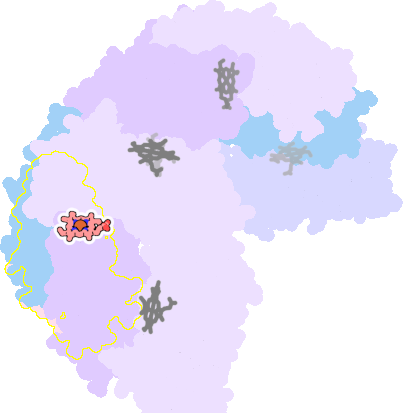 |
sad. -0.40 ruf. +0.07 dom. +0.09 bre. -0.55 |
MET | chainID: A, resSeq: 52, molecule: BACTERIOFERRITIN |
oxidoreductase oligomeric count: 24 pocket vol.: 538.0 Å3 d(Fe-oop): 0.063 Å |
| MET | chainID: B, resSeq: 52, molecule: BACTERIOFERRITIN |
|||
3e1q-D-200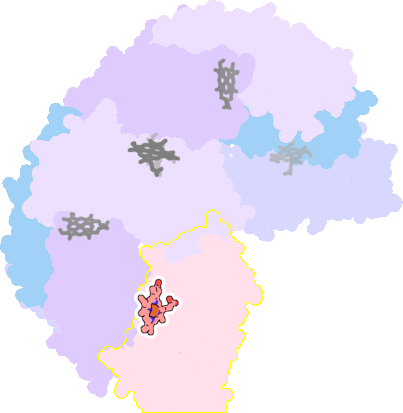 |
sad. -0.41 ruf. +0.08 dom. +0.08 bre. -0.55 |
MET | chainID: C, resSeq: 52, molecule: BACTERIOFERRITIN |
oxidoreductase oligomeric count: 24 pocket vol.: 518.0 Å3 d(Fe-oop): 0.069 Å |
| MET | chainID: D, resSeq: 52, molecule: BACTERIOFERRITIN |
|||
3e1q-F-200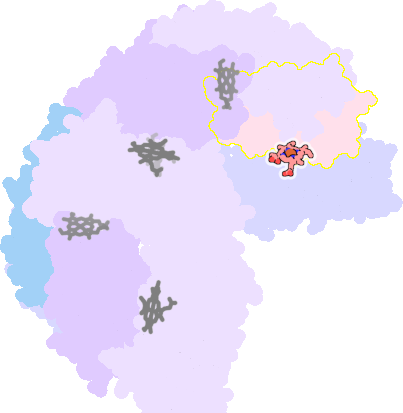 |
sad. -0.41 ruf. +0.07 dom. +0.09 bre. -0.55 |
MET | chainID: E, resSeq: 52, molecule: BACTERIOFERRITIN |
oxidoreductase oligomeric count: 24 pocket vol.: 545.0 Å3 d(Fe-oop): 0.042 Å |
| MET | chainID: F, resSeq: 52, molecule: BACTERIOFERRITIN |
|||
3e1q-I-200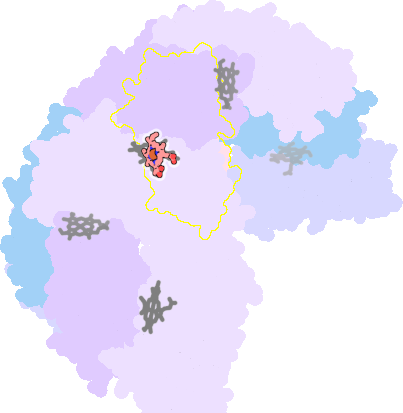 |
sad. -0.42 ruf. +0.08 dom. +0.10 bre. -0.55 |
MET | chainID: I, resSeq: 52, molecule: BACTERIOFERRITIN |
oxidoreductase oligomeric count: 24 pocket vol.: 547.0 Å3 d(Fe-oop): 0.035 Å |
| MET | chainID: J, resSeq: 52, molecule: BACTERIOFERRITIN |
|||
3e1q-L-200 |
sad. -0.40 ruf. +0.08 dom. +0.09 bre. -0.56 |
MET | chainID: K, resSeq: 52, molecule: BACTERIOFERRITIN |
oxidoreductase oligomeric count: 24 pocket vol.: 542.0 Å3 d(Fe-oop): 0.056 Å |
| MET | chainID: L, resSeq: 52, molecule: BACTERIOFERRITIN |
|||
