Browser
Protein data
function: oxidoreductaseexperiment: X-RAY DIFFRACTION
resolution: 2.8 Å
origomeric count: 24
axial ligand #1: MET
chainID: A,resSeq: 52,
Coordination distance[Å]: 2.231
molecule: BACTERIOFERRITIN
Organism: ESCHERICHIA COLI
axial ligand #2: MET
chainID: B,resSeq: 52,
Coordination distance[Å]: 2.335
molecule: BACTERIOFERRITIN
Organism: ESCHERICHIA COLI
List of other hemes in pdb:3e1n
| ID of heme | Distortion | Axial ligands on heme | Function & structure | |
|---|---|---|---|---|
3e1n-D-200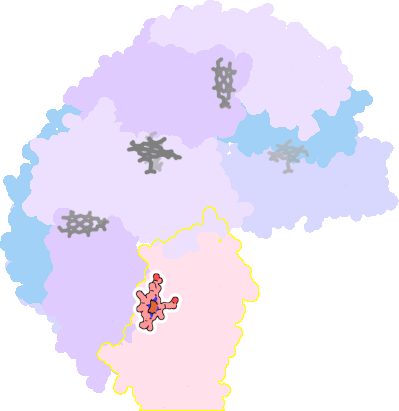 |
sad. -0.30 ruf. +0.01 dom. +0.28 bre. -0.40 |
MET | chainID: C, resSeq: 52, molecule: BACTERIOFERRITIN |
oxidoreductase oligomeric count: 24 pocket vol.: 536.0 Å3 d(Fe-oop): 0.053 Å |
| MET | chainID: D, resSeq: 52, molecule: BACTERIOFERRITIN |
|||
3e1n-F-200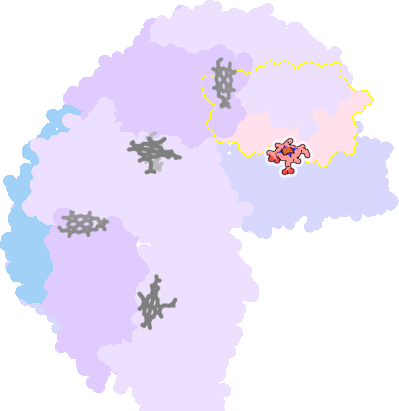 |
sad. -0.33 ruf. +0.01 dom. +0.03 bre. -0.40 |
MET | chainID: E, resSeq: 52, molecule: BACTERIOFERRITIN |
oxidoreductase oligomeric count: 24 pocket vol.: 532.0 Å3 d(Fe-oop): 0.003 Å |
| MET | chainID: F, resSeq: 52, molecule: BACTERIOFERRITIN |
|||
3e1n-G-200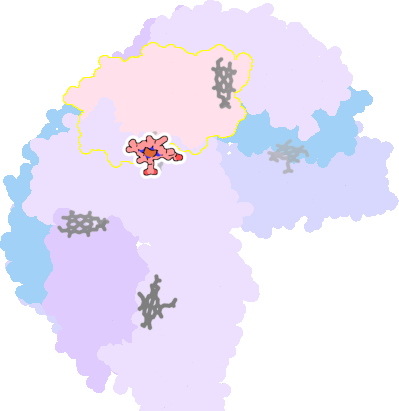 |
sad. -0.19 ruf. +0.25 dom. +0.13 bre. -0.40 |
MET | chainID: G, resSeq: 52, molecule: BACTERIOFERRITIN |
oxidoreductase oligomeric count: 24 pocket vol.: 545.0 Å3 d(Fe-oop): 0.008 Å |
| MET | chainID: H, resSeq: 52, molecule: BACTERIOFERRITIN |
|||
3e1n-I-200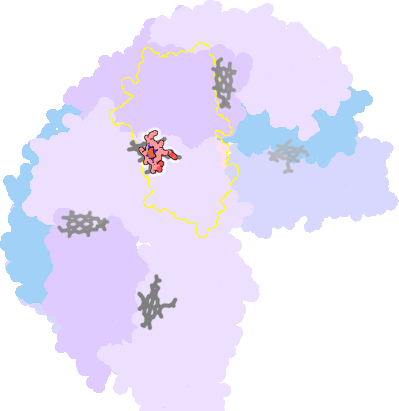 |
sad. -0.43 ruf. +0.26 dom. +0.08 bre. -0.39 |
MET | chainID: I, resSeq: 52, molecule: BACTERIOFERRITIN |
oxidoreductase oligomeric count: 24 pocket vol.: 544.0 Å3 d(Fe-oop): 0.008 Å |
| MET | chainID: J, resSeq: 52, molecule: BACTERIOFERRITIN |
|||
3e1n-L-200 |
sad. -0.30 ruf. +0.09 dom. +0.13 bre. -0.41 |
MET | chainID: K, resSeq: 52, molecule: BACTERIOFERRITIN |
oxidoreductase oligomeric count: 24 pocket vol.: 548.0 Å3 d(Fe-oop): 0.044 Å |
| MET | chainID: L, resSeq: 52, molecule: BACTERIOFERRITIN |
|||
